According to its own words in February 2024 “Health Canada is responsible for helping Canadians maintain and improve their health. It ensures that high-quality health services are accessible, and works to reduce health risks.”
In HC’s mission, vision and values page, it reads “helping the people of Canada maintain and improve their health.” “guided by our personal integrity”, “communicate honestly and effectively”, “advance the public good” and “provide credible information”.
In this post, I address just four of HC’s many failures:
· COVID-19 vaccines,
· Agile Licensing or Facilitating Licensing of patentable drugs
· Impairing Accessibility of non-patentable drugs
· Not informing citizens about health maintenance with vitamin D (it was ivermectined)
COVID-19 (C19) vaccines will “stop the spread” and are “safe and effective”; this was the mantra that was drummed into the ears of the Canadian public for months before and after the “interim order”.
Many writers have analyzed the data with respect to the mRNA vaccines, and found them to be neither effective nor safe. I have written about this in an early entry- https://nakatsu.substack.com/p/safe-and-effective and will not repeat it in detail here. Suffice it to say that HC failed to ensure that they were safe and were effective.
The problems with the vaccines are described well in Mead MN, Seneff S, Wolfinger R, Rose J, Denhaerynck K, Kirsch S, McCullough PA. COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign. Cureus. 2024 Jan 24;16(1):e52876. doi: 10.7759/cureus.52876. Retraction in: Cureus. 2024 Feb 26;16(2):r137. PMID: 38274635; PMCID: PMC10810638.
Then the paper was censored “The Editors-in-Chief have retracted this article. Following publication, concerns were raised regarding a number of claims made in this article. Upon further review, the Editors-in-Chief found that the conclusions of this narrative review are considered to be unreliable due to the concerns with the validity of some of the cited references that support the conclusions and a misrepresentation of the cited references and available data. The authors disagree with this retraction.” Fortunately, “PMC will not remove retracted articles from its archive.” so it should be available to readers indefinitely.
I am led to believe that the editors (John R. Adler Jr., Alexander Muacevic) acted on “orders from above”, suggesting a monetary motive. They should have refused, especially given the thorough critiques of eight referees.
In addition, the “vaccines” were modified RNA wrapped in a fatty protective layer- i.e. a modified RNA comprising unusual N-methyluridine, (modRNA- within a lipid nanoparticle). Such products should have been regulated as genetic therapies rather than vaccines. It amazes me that so many people who eschew GMO foods (genetically modified organism) are keen to have me and others injected with modRNA. Since I wrote my previous criticism of the modRNA, I have learned that they are contaminated with DNA and some in some batches the purity is barely 50%.
HC gave the “modRNA vaccines” approval to be injected into the arms of Canadians based on a truncated phase 3 clinical trial, which showed no saving of lives. In fact, after using the most generous assumptions, Mead et al calculated that the injections resulted in 14 times more deaths than they “saved”.
What HC didn’t tell us was that the “vaccines” could distribute throughout the body. Instead we were told that they stayed at the injection site and were destroyed in hours. These are just some of the problems with the “modRNA vaccines” that HC overlooked while failing to help Canadians “maintain their health.”
Did HC communicate honestly and effectively? Did it provide credible information?
For starters communication is a two-way street, meaning that HC says that information would given to and received from citizens. Part of the safety characteristics of a vaccine requires collecting and receiving information about adverse effects. Up to Dec 3, 2023, 105,016,456 vaccine doses had been administered and this resulted in 11,702 reports of serious adverse events (0.011% of all doses administered). I suggest that this rate of serious adverse effects is at least a one-thousand-fold under-estimate. Why? you might ask.
Consider that the US Vaccine Adverse Effects Reporting System (VAERS) is widely known and is accessible for public reporting; even VAERS has been suggested to capture only 1% of USA adverse effects of the COVID-19 vaccines. Our Canadian system is not widely known, and its structure discourages reception of adverse effect reports. Healthcare providers are instructed to complete a nine-page form to report any one adverse effect. If any of the details in the form is questionable, the report can be (and usually is) rejected by the local office of public health. For example, one MD told me that 6 of 6 reports that he submitted were rejected locally because of a single omission or error; then he was not given the opportunity to correct the form and re-submit it. Examination of page 3 of the form shows the level of detail that could give rise to rejections.
Imagine that you are a doctor and you are interviewing a patient with a cardiac problem, such as atrial fibrillation two days after injection of modified RNA. What is the likelihood of the patient knowing the Lot number and Expiry date? This MD told me that his offer to correct the form was rejected.
To me, it’s amazing that even 11,702 serious adverse effect reports were recorded.
Conclusion- HC failed to communicate effectively with doctors and patients, especially those who experienced adverse effects.
Facilitating Licensing of patentable drugs. “Agile regulations” is another name. HC is amending the Food and Drug Regulations (FDR) taking advantage of “recent experience with regulatory agilities successfully piloted through the COVID-19 interim orders and their transition to regulations.” HC’s failure to ensure that the “vaccines” for COVID-19 were “safe and effective” does not instill confidence in its using “recent experience” wisely.
Under Agile Regulations a novel drug could be licensed and sold in Canada after reduced pre-approval testing for efficacy and toxicity. HC plans to balance this reduction with enhanced post-marketing surveillance or phase 4 studies. This moves much/most of the toxicity testing from phase 3 clinical trials to experience by the general public. While this would help manufacturers and some consumers by allowing new drugs to be available earlier, it comes with the risk of exposing many more consumers to toxicity.
Although the C19 vaccines were approved in Canada under “interim order”, it could have been called “agile regulations”. Thus, with very brief and truncated phase 3 trials, these novel “drugs” were introduced to Canadians.
What has our experience been with phase 4 surveillance?
In a word- miserable. HC has not established a robust means for recording all adverse effects, suspected and/or real. Given the publicity afforded to the dangers of C19, you could be forgiven for expecting an analogous effort aimed at recording adverse effects of the vaccines. To this writer, it appears that HC failed to publicize its vaccine adverse effects reporting system, and made it as difficult as possible for doctors and the public make reports.
How would you grade HC’s performance herein. Pass? Or Fail?
Impairing Access to Natural Health Products
There is a possibility that some people may denigrate natural health products (NHP) as being unworthy of serious consideration because they are impure, often of unknown composition and often based on folklore rather than modern science. Be that as it may, it’s worthwhile remembering that the majority of patented drugs arose from extracts of plants or animals. Even the ACE inhibitors got their start from an extract of snake venom; the first such drug, captopril, was followed up with rational drug design to result in other patented drugs such as enalapril. Thus natural health products must be considered as serious treatments for various ailments and citizens should have access to them.
Now HC proposes to limit access to NHPs and citizens aren’t being provided with information in a timely manner. In fact, one might say that government has made these proposals for change in most surreptitious manner. The Liberal-NDP coalition passed bill C-47, which was basically an omnibus budget bill; but it contained sections 500-504 in which changes to regulations regarding NHPs were described; as bill C-47 contained 681 sections, the NHP segment was effectively hidden and protected from debate. It should be noted that these same ideas were floated previously as bill C-51 in 2008; this bill was stopped by thousands of citizens who mailed wheelbarrow loads of opposition to parliament.
Now there are people, many of whom opposed the legislation when it was presented as C-51, who are working to have sections 500-504 of C-47 repealed. This action is led by Shawn Buckley of The Natural Health Products Protection Association (NHPPA, https://nhppa.org) and is based on these key issues:
Health Canada is implementing stricter regulations that will significantly impact consumer choice and restrict access to health products and supplements.
Health Canada is introducing new fees on natural health products, which will place a considerable financial strain on NHP businesses. This will lead to significant price hikes for consumers or even force many small to medium-sized companies out of business.
Increased Health Canada censorship will prevent truthful advertising by both manufacturers and natural health stores.
Huge new fines will cripple and destroy the natural health community. Prior to June 22, 2023, the maximum fine you faced for violating the laws on NHPs (such as selling a product with improper labelling) was $5,000. Now it is $5,000,000 a day.”
Remember, HC stated it would “communicate honestly and effectively”. So burying these changes in an omnibus was “honest communication”?
HC should have communicated with those who could be affected by such legislation- citizens who buy NHP and businesses that sell NHP and manufacturers that produce NHP and practitioners who recommend NHP and farmers who grow NHP. These regulatory changes should have been debated openly, and evidence should have been reported and examined in detail. To impose these regulatory changes under the “cover of darkness” is cowardly at best and flies in the face of democracy.
To this point, I have raised HC’s failure of commission; let’s examine its failures of omission.
Protection from Responsibility. HC omitted holding vaccine manufacturers responsible for shortcomings of the products; they cannot be sued for any damage caused by the adverse effects of modRNA “ vaccines” or for their lack of effectiveness. This removed all incentives for the manufacturers to ensure the safety and effectiveness of their products. But it gave them a great incentive to market them as soon as possible. Had this accountability been maintained, the vaccine sellers would have protected themselves from criminal prosecution and law suits by acting much more responsibly.
Vitamin D. HC did little or nothing to promote health maintenance of the populace, especially as it applies to immune function. In fact, most of its messages suggested actions that would impair not bolster immune functions. We were told to go home and isolate. We were encouraged to stay indoors depriving ourselves of fresh air, sunshine and exercise. Gymnasia were closed, golf courses were closed, public parks were closed. Moreover, HC did not promote healthy food and appropriate nutrients in any meaningful manner.
This was most egregious in the case of vitamin D, which now has been documented as being essential for many cellular functions including those associated with protection against infections, both viral and bacterial. (see https://dgreatbiologyreset.com to access a free book by Grimes & Anderson- Vitamin D3 and the Great Biology Reset).
Failure in HC came right from its leadership. In response to a question about the potential benefits of promoting vitamin supplementation by Independent MP Derek Sloan, then Health Minister Patty Hajdu said that it was "fake news".
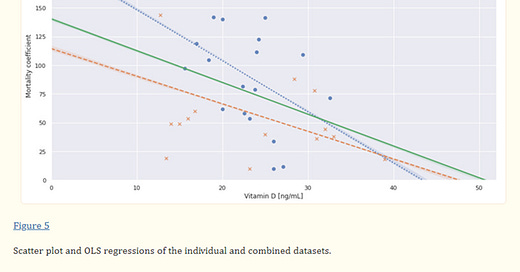
Compare this to some of the information revealing that vitamin D supplementation could have protected the public from C19 in the manner that the vaccines were hoped and hyped. The graph below shows that maintenance of serum vitamin D levels at ~50 ng/mL or 125 nMol/L virtually eliminated mortality due to C19 (Borsche et al, 2021 https://pubmed.ncbi.nlm.nih.gov/34684596/ ).
Ivermectin for C19 was ivermectined.
Many things were suppressed during C19. But ivermectin stands out in my mind as being the poster child of suppression- so much so that one might state that free speech was “ivermectined”.
From my reading early in C19, ivermectin was an effective antiviral drug with efficacy against C19 infection and excellent safety. See https://c19ivm.org for ivermectin references and summaries, and vigiaccess.org for toxicity reports. Thus, HC was asked about ivermectin by many parties but it did nothing to make ivermectin available to doctors to prescribe and citizens to use. Surely HC had scientists capable of objectively documenting the potential of ivermectin to prevent and treat C19 within months of its arrival in Canada. HC claimed that it could not or would not act unless a manufacturer requested the right to market it. Surely HC’s commitment to “advance the public good” commits it initiate some actions to protect the health of citizens.
You have to ask yourself- Was HC acting on behalf of Canadians or pharmaceutical companies?
It’s pretty clear now that existing (and inexpensive) drugs could have and would have prevented disease and death throughout the entire population of the world. Expensive novel modRNA injections and antivirals were unnecessary. Many medical practitioners and investigators have stated that modRNA did more harm than good.
Given all the failures at HC, what should be done? What are the solutions?
First of all, HC must stop catering to the pharmaceutical industry. Just because much regulatory budget comes from fees paid by drug companies doesn’t mean that HC has to cater to them. Think about it. Hunters pay for hunting licenses but the provinces don’t provide shooting stations. In fact, they limit the harvest via game tags, which are often granted by lottery. Funding drug approval by a tax on prescription drugs would reveal who pays for drug regulation; currently citizens pay indirectly through higher drug prices charged by manufacturers.
Secondly, HC must be distanced from political influence. I realize that it will be impossible to erect an impenetrable wall but we should strive to restrict political interference. This might be done by creating a crown corporation to oversee licensing of drugs and device. The corporation could be established with a board of directors answerable to the citizens by an independent appointment body. Independence will require just some imagination.
Thirdly, consider some suggestions from people such as Joel Lexchin who has published extensively in the field. Quotation “These could include public funding of clinical trials . . . and paying companies a monetary reward that reflects the social value of new medications in return for the companies surrendering their monopoly patent rights . . . . Governments need to put more weight on protecting public health and reducing wasteful spending on drugs with no therapeutic advantages over existing products, and less on protecting the interests of the pharmaceutical industry. Doing so will benefit both patients and the public purse.” https://www.longwoods.com/content/25195/the-pharmaceutical-industry-and-the-canadian-government-folie-deux